Guiding question: How can we model the particulate nature of matter?
Table of Contents
Matter and its types
Matter: something that has mass, volume and occupies space.
There are two types of matter; pure substances which are elements and compounds, and mixtures which are either homogenous or heterogenous.
- Elements contain only one type of atom and cannot be broken down chemically into simpler substances.
- Compounds contain two or more different types of atoms chemically combined in fixed ratios.
- There are 92 naturally occurring elements in the periodic table.
- The most recent element synthesized is Nihonium.
- Elements occur as individual atoms.
eg. copper
or can occur as molecules
eg. H2 - Compounds can be broken down into their elements by chemical methods.
- Elements lose their properties while compounds are formed, the compounds show different properties from their elements.
- Mixtures contain two or more elements or compounds.
- Mixtures are not chemically bonded.
- Mixtures can be separated by physical method.
- Elements and compounds retain their properties in a mixture.
- Heterogenous mixtures are non-uniform compositions.
- Heterogenous mixtures have different visible phases.
eg. oil + water - Homogenous mixtures have uniform composition.
- Homogenous mixtures have a single phase.
eg. salt + water
Here are a few more examples of homogenous, heterogenous and properties of mixtures:
| Mixture | Homogenous or Heterogenous |
| Air | Homogenous |
| Bronze (alloy) | Homogenous |
| Concrete | Heterogenous |
| Orange juice with pulp | Heterogenous |
| Mixture | What technique can be used to separate the components? | The property that is different in the components |
| Air | Homogenous | Boiling point |
| Salt and Sand | Homogenous | Solubility in water |
| PIgments in food colors | Heterogenous | Adsorption on cellulose |
| A iron-sulfur mixture | Heterogenous | Magnetism |
Separation techniques
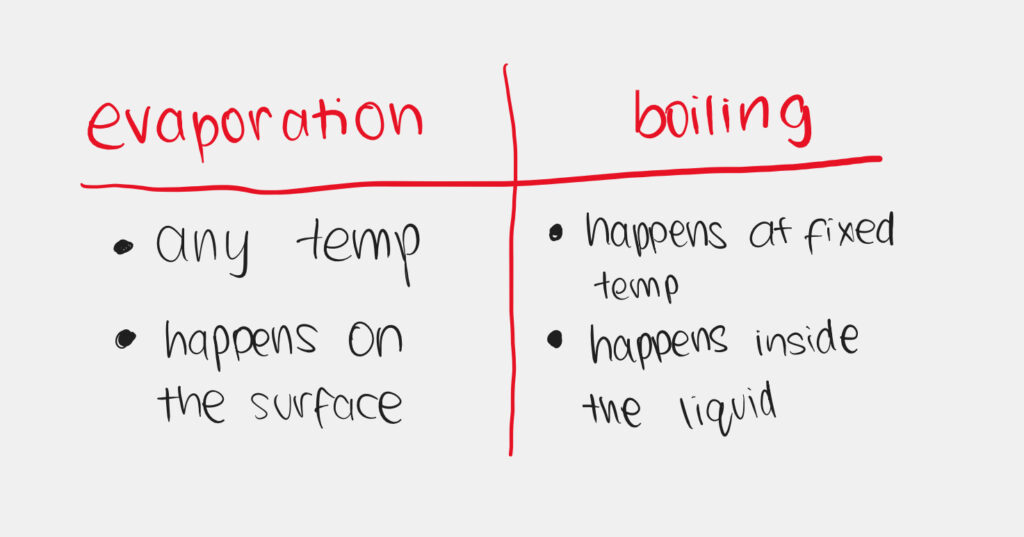
Evaporation
- It is used to separate a mixture in which a solute is dissolved in a solvent like water.
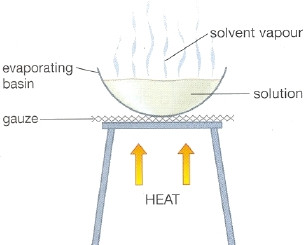
- The solid which was dissolved, can be separated by evaporation.
- The solvent evaporates into the surroundings.
Filtration
- It is used to separate an insoluble solid from a liquid or a solution.
- The insoluble solid after the filtration is called residue and the separated solution is called filtrate.
Solvation
- It is used to separate a heterogeneous mixture of two solids based on the difference in their solubilities. Usually one of the solids get dissolved while the other remains insoluble which can be further separated by filtration.
Distillation
- It separates mixtures of miscible liquids with differences in volatility and boiling points.
eg. ethanol and water
Recrystallization
- It is used to remove impurities mixed with the solid.
- This technique works on the various solubilities of solids at different temperatures.
- In this technique an impure mixture is first dissolved in a hot solvent.
- Insoluble will be filtered off at this point.
- The solution is allowed to cool, crystals start forming and then can be filtered.
eg. It is used to purify sugar crystals from sugarcane juice and in pharmaceutical industries to remove impurities from medicines.
Paper Chromatography
- It is used to separate a mixture of solutes in a solvent.
- There are two phases;
1. Mobile phase
2. Stationary phase - It works based on the different solubilities of solutes in a solvent.
- The spot at the top has the maximum affinity towards mobile phase and the bottom spot has the most affinity towards stationary phase.
States of matter and changes of state
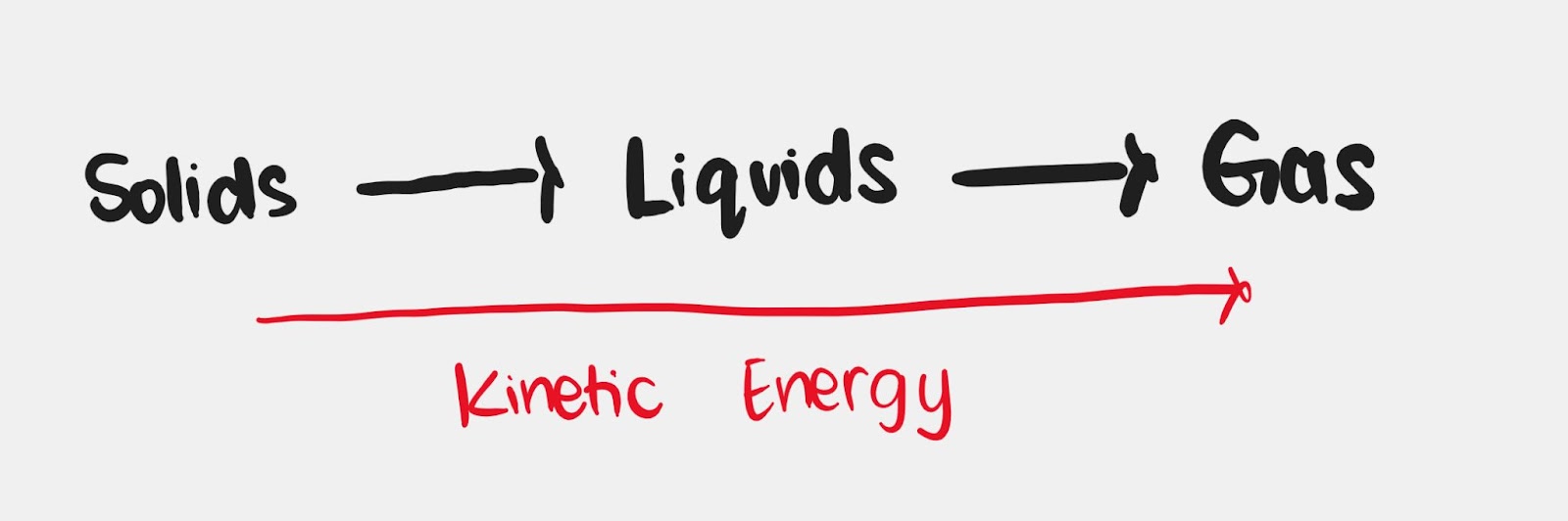
Kinetic molecular theory (KMT)
- Matter is made up of small particles.
- They have kinetic energy which makes them move.
- The amount of kinetic energy is proportional to the temperature, particles have greater motion at high temperature or vice versa.
- Collisions between particles bring no loss in kinetic energy (elastic).
Sublimation: is a change of state from solid to gas
Deposition: is a change of state from gas to solid
Vapourization: evaporation and boiling together
Temperature and Kinetic energy
heating and cooling curve
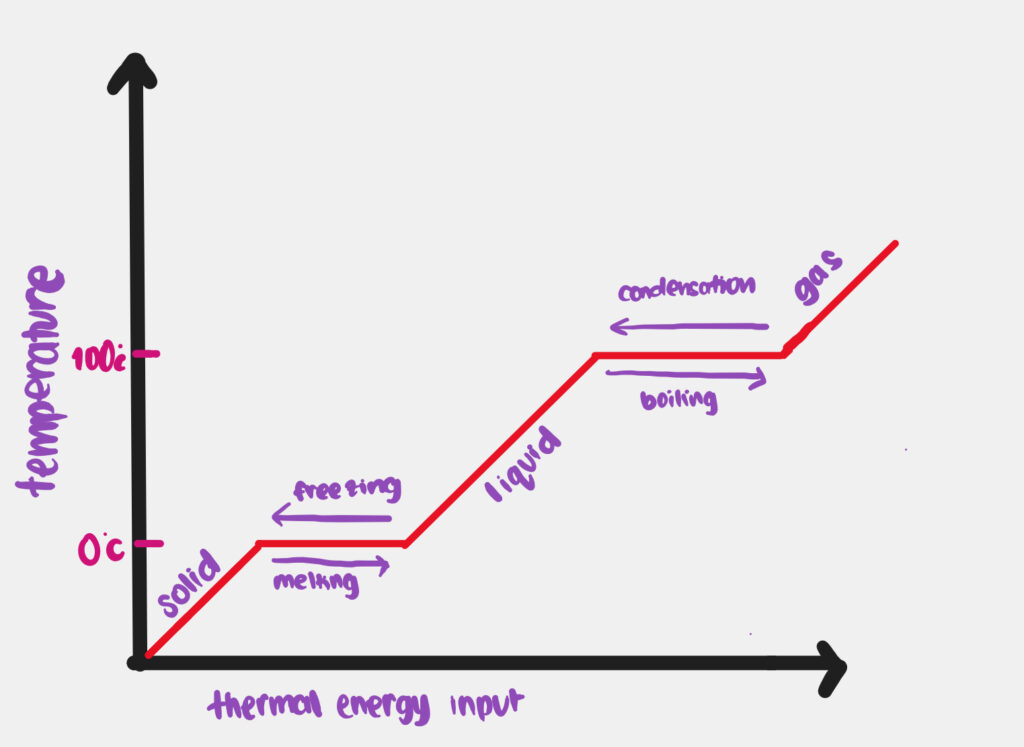
- The temperature remains constant during melting and boiling as the added heat is used to overcome the intermolecular forces between the particles.
Questions
S1.1 is a newly added chapter into the 2025 syllabus so there are no past paper questions on it. Hence, I am adding a few questions of my own. Some taken from external resources.
- MCQ: Which equation does not represent sublimation or deposition?
a) NH3(g) + HCl(g) –> NH4Cl(s)
b) I2(s) –> I2(g)
c) C16H10O(s) –> C16H10O(g)
d) H2O(g) –> H2O(s) - MCQ: The order in which oxygen, methane, nitrogen and argon will diffuse from slowest to fastest, at the same conditions of temperature and pressure is:
a) O2 ﹤ N2 ﹤ Ar﹤ CH4
b) CH4 ﹤ Ar﹤ N2 ﹤ O2
c) N2 ﹤ O2 ﹤ Ar﹤ CH4
d) Ar﹤O2 ﹤ N2 ﹤ CH4 - Structured: The melting point of benzene is 5.5°𝐶, and its boiling point is 80.1°𝐶.
a) Draw the heating curve for benzene from -10°𝐶 to 110°𝐶.
b) State the state of matter of benzene at -6.0°𝐶.
c) Describe the arrangement of benzene molecules at 50.0°𝐶.
d) 5.00 𝑔 of NaCl is added to 100 𝑐𝑚3 of benzene. The salt is insoluble in the solvent.
i. Determine the type of mixture formed.
ii. Suggest a technique that can be applied to separate and recover benzene and sodium chloride. Sketch a labeled diagram to show how the separation will take place.
Answers to questions
- A
Because it is a chemical reaction occuring and not a state change - D
The order by molecular mass from lightest to heaviest is CH4 ﹤ N2 ﹤ O2 ﹤ Ar. Therefore, from slowest to fastest will be the opposite order Ar ﹤ O2 ﹤ N2 ﹤ CH4 - a) Temperature must be on the vertical axis and time on the horizontal axis;
Positive gradient from -10.0°𝐶 until 5.5°𝐶 and a flat section at 5.5°C;
Positive gradient from 5.5°𝐶 until 80.1°𝐶 and a flat section at 80.1°C and positive gradient beyond 80.1°𝐶;
b) solid
c) Any 2 of the following:
– Molecules close to one another (not in a regular pattern);
– Molecules vibrating, rotating, and moving around;
– Molecules can slide over each other.
d) i. Heterogenous mixture
ii. Filtration
A funnel with filtering paper must be shown in the sketch;
Sodium chloride/residue in the filtering paper;
Benzene/filtrate shown.

external resources used:
1. my chem notebook
2. save my exams notes
other chem blogs i have written:
1. Ionic Bonding