This article provides all information needed for Topic 4: The Chemicals of Life.

Topic Aims of Topic 4: The Chemicals of Life
- Understanding the importance of water
- Composition of carbohydrates, lipids and proteins are made of
- Roles of these biological molecules in living things
- Food tests
- Structure of DNA
The Importance of Water as a Chemical of Life
- All organisms consist of 80% water. This is because:
- Cytoplasm that fills all cells are made of water. This watery part is called the cytosol.
- The intracellular fluid that forms the surroundings of cells are also mostly water.
- Metabolic reactions inside of organisms can only occur with the presence of water because it acts as an important solvent (dissolves the reactants to form chemical reactions).
- These metabolic reactions sustain life.
- Water is needed to form plasma, the watery part of the blood that dissolves nutrients in the blood to transport these nutrients throughout the body.
- Water dissolves nutrients and enzymes in the alimentary canal for absorption of nutrients.
- Water dissolves urea formed from digested proteins which we excrete as waste.
Composition of Carbohydrates, Lipids and Proteins
Carbohydrates (CHO)
Carbohydrates are made out of carbon, hydrogen and oxygen. Carbohydrates are usually made out of two parts hydrogen and one part oxygen or carbon atoms, e.g. C6H12O6 as glucose. They are also called sugars.
Monosaccharides
- These are the simplest sugars like glucose. The image below shows the structure of glucose, which are six carbon atoms forming a circle, with oxygen and carbon at the ends.
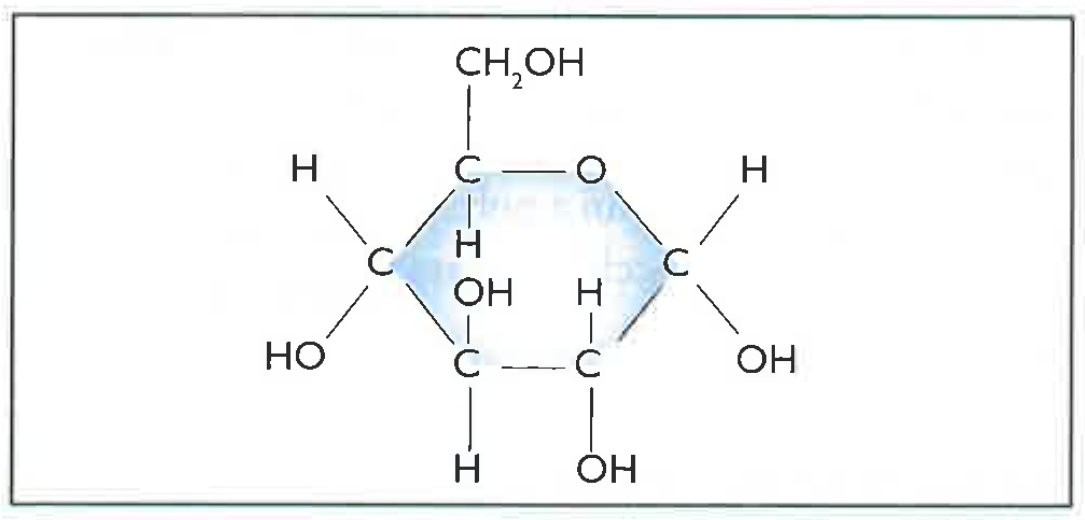
- Simple sugar molecules are small, soluble in water and taste sweet.
Disaccharides
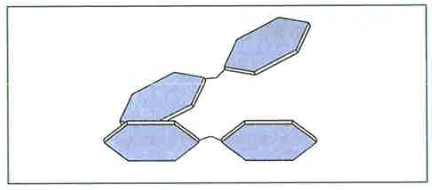
- If two of these simple sugar molecules bind together, they form a disaccharide. Some examples include sucrose, maltose and galactose.
- These are also soluble in water and taste sweet.
Polysaccharides
- When many of these simple sugars bind together, then they form a polysaccharide.
- These are hundreds and thousands of simple sugars that form into a chain.
- Some examples include cellulose found in plant cells and starch found in plant cells. Animal cells have glycogen stored in them instead of starch.
- Polysaccharides are insoluble and do not taste sweet.
Lipids (CHO)
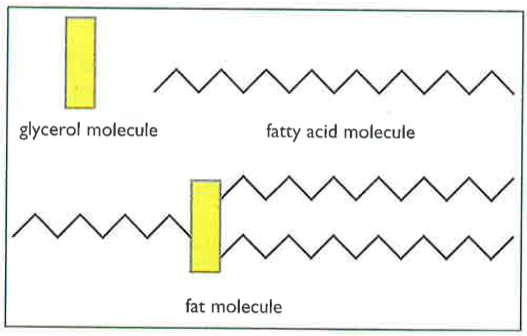
Lipids are fats. Lipids are insoluble in water and those that are liquid at room temperature are called oils. They contain carbon, hydrogen and oxygen, just like carbohydrates; however, a fat molecule differs in structure. Fat molecules have glycerol heads and three fatty acid tails.
Proteins (CHONS)
Proteins are made out of carbon, hydrogen and oxygen but they are also made of nitrogen and sulfur. Proteins are made out of smaller chains of molecules called amino acids. These amino acids are formed in many sequences to form the 20 different types of protein found in the human body.
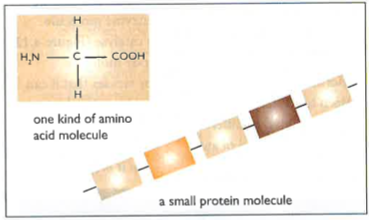
Each specific sequence of amino acids curl up into different shapes suited for their use. These help them with their function. For example, the way a protein curls up makes it suitable to be an enzyme, as the molecule it reacts with fits perfectly into it; and antibodies have specific shapes to attach to the antigens. Additionally, some proteins are soluble in the body, like haemoglobin; while some are insoluble, like keratin.
Roles of These Chemical Molecules in the Body
Carbohydrates
- Provides energy, releasing 17 kJ during respiration
Lipids
- Provides energy, releasing 39 kJ during respiration
- Most cells use carbohydrates first then lipids after all carbohydrates have been used
- The extra energy provided makes lipids suitable for energy storage
- When lipids are stored in specific cells, they form adipose tissue
Proteins
- Used to make new cells for growth
- Used to make antibodies that kill pathogens which enter the organism
- Used to make enzymes which quicken many metabolic reactions in the body
Food Tests for the Chemicals of Life
Carbohydrates
Sugars
When testing for sugars, a reagent called Benedict’s solution is used. If the sample tested contains reducing sugars, then the Benedict’s solution will turn into different colours: blue for no sugar, green for low concentration of sugar, yellow/orange for a normal concentration of sugar and red for a high concentration of sugar.
Here are the steps for the Benedict’s test:
- Prepare food samples into test tubes
- Prepare water bath of 95°C
- Add 3-5 drops of Benedict’s solution
- Place test tubes into water bath for approximately 3 minutes.
- Observe the changes in colour.
Starch
The test for starch is far easier than the test for reducing sugars. All we need to do is to add an iodine solution to the sample. If it turns blue-black, then starch is present. If it remains brown, then starch is absent.
Lipids
The food test for lipids is called the ethanol emulsion test. For the end result, if the solution is cloudy, then lipids are present. If the solution remains clear, then lipids are absent.
Here are the steps for the ethanol emulsion test:
- Mix the sample with ethanol
- Shake, then let it rest for 2 minutes
- Pour distilled water into the test tube containing the sample
- Observe results
Proteins
The test for proteins is called the biuret test. For the end result, if the solution turns purple, the protein is present. If the solution remains blue, then proteins are absent.
Here are the steps for the biuret test:
- Prepare the sample
- Add 3-5 drops of copper sulfate solution
- Add gently add dilute potassium hydroxide
- Observe the colour change.
Structure of DNA
DNA means deoxyribonucleic acid and it is the molecule which represents our characteristics we inherited from our parents. A DNA molecule is formed from two DNA strands twisted in a helical shape. On each strand, there are bases peeking from one of the edges of them.
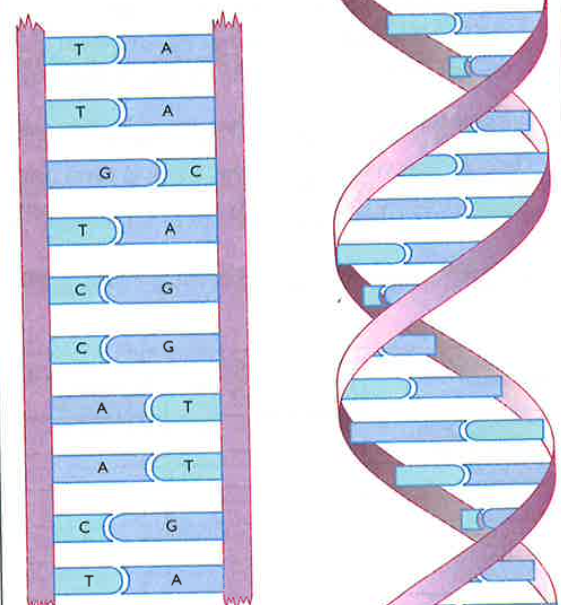
There are four types of bases:
- A for adenine
- T for thymine
- C for cytosine
- G for guanine
In all situations A and T always bond together while C and G always bonds together. The sequences in our DNA determine how amino acids are sequenced. This determines how our proteins are formed, which creates our tissues, our organs and ourselves.
That’s it for Topic 4: The Chemicals of Life! If you’d like more resources on this topic, check out this link!


