Enthalpy is a concept used in thermodynamics in IBDP Chemistry (Especially in Energetics) to represent the heat content or thermal energy of a system. In simple terms, the enthalpy of a system represents the total amount of energy it contains, which can be utilized when the system exchanges heat with another system or performs work. This portion is important for both Sl and Hl
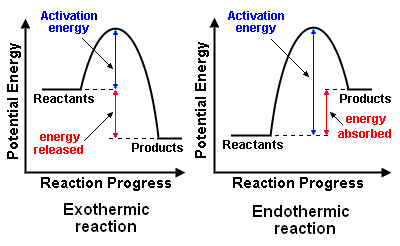
Enthalpy is primarily used to describe the flow of heat in chemical reactions or phase changes. For instance, if a reaction releases heat, it’s called an exothermic reaction, and the change in enthalpy (ΔH) is negative. Conversely, if a reaction absorbs heat, it’s known as an endothermic reaction, and the change in enthalpy is positive.
To make the concept of enthalpy easier to understand, let’s consider the example of boiling water. When we boil water, we transfer energy (usually in the form of heat) to the water. This energy increases the water’s enthalpy, leading to a phase change where water turns into steam at a certain temperature. During this process, the water absorbs heat, making it an example of an endothermic process.
Temperature VS Heat in terms of Energetics
| temperature | heat |
| Heat is the total energy (kinetic and potential) of the molecular motion in a substance. | Temperature is a measure of the average kinetic energy of the molecules in a substance. |
| Heat is measured in joules (J), calories (cal), or British Thermal Units (BTU). | Temperature is measured in degrees Celsius (°C), Fahrenheit (°F), or Kelvin (K). |
| Heat depends on the mass of the substance, its specific heat, and the amount of temperature change. | Temperature does not depend on the mass or size of the object. It is a measure of how hot or cold an object is. |
Imagine you’re at a concert. The temperature in this analogy is like the average energy or mood of the crowd. Just as a few really enthusiastic fans can raise the average energy level of the crowd, a few fast-moving molecules increase the temperature of a substance.
Heat, on the other hand, is akin to the total energy or excitement generated by the entire crowd. It doesn’t matter if the concert is in a small venue with a handful of highly enthusiastic fans or a giant stadium with thousands of people; the total energy can be immense in both, but it’s the size of the crowd (akin to the mass of the substance and the amount of substance present) that determines the total amount of energy or “heat” present.
Types of systems in IBDP chemistry
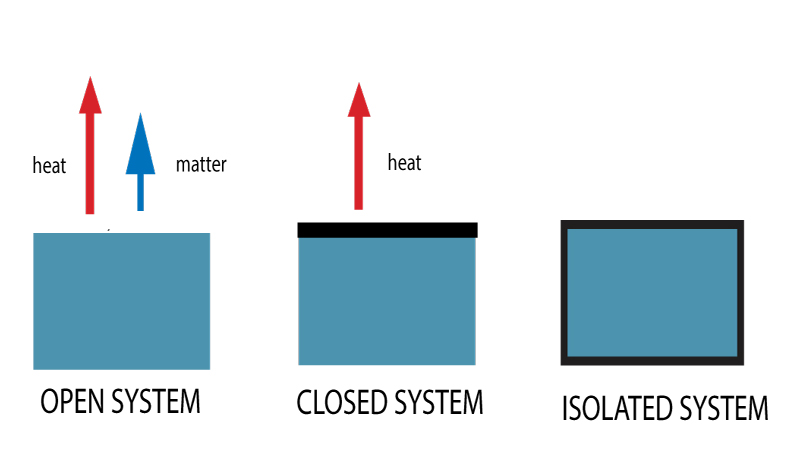
Open system:Open systems can exchange both energy and matter with their surroundings. This type of system is most common and relevant in real-world scenarios. An example of an open system is a boiling pot of water without a lid. The pot exchanges heat with its surroundings, and water vapor escapes into the air, demonstrating both energy and matter exchange.
Closed systems:A closed system can exchange energy (in the form of heat or work) but not matter with its surroundings. The mass of a closed system remains constant, but its energy can change. An example of a closed system is a sealed pot of boiling water on a stove. The pot can transfer heat to the surroundings (and possibly receive heat if the stove is on), but the water and steam inside the pot cannot escape.
Isolated systems:Open systems can exchange both energy and matter with their surroundings. This type of system is most common and relevant in real-world scenarios. An example of an open system is a boiling pot of water without a lid. The pot exchanges heat with its surroundings, and water vapor escapes into the air, demonstrating both energy and matter exchange.
alright! thx for joining my chemistry journey and you can check out more of our blogs here:https://prodatblog.org/e