Introduction
What are carbohydrates?
Well, in our body we need different types of nutrients. Among them are lipids, amino acids and one of them are carbohydrates.
As the name suggest CARB-O-HYDRATE, it is a compound that contains Carbon, Oxygen and Hydrogen. They are bonded through covalent bondings thanks to carbons 4 valence electrons. When the carbohydrates are broken down the bonds break leading to release of energy.
There are different types of carbohydrates:
- Monosaccharides: They are the simplest among the three. Meaning they only consist of one sugar molecule. Hence, they cannot be further broken down. The examples of monosaccharides are glucose, fructose and galactose.
Now, one very important thing to note is that in IB biology you are required to distinguish between the two types of glucose, even requiring you to draw them. There are two different glucose that are discussed in the IB one is Alpha dextrous glucose and Beta dextrous glucose.
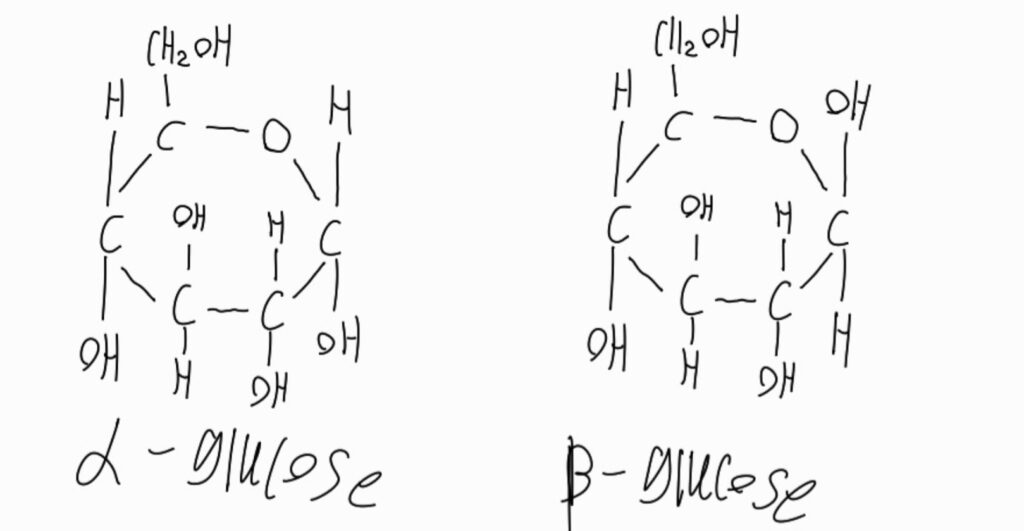
The main difference is that beta glucose have their OH group upwards while Alpha glucose OH groups are downwards.
2. Disaccharides: They are a carbohydrate consisting of two monosaccharide bonded together by a condensation reaction (a reaction that gives off water as a by product). In IB exams they will ask you to memorize three types of disaccharides which are lactose, sucrose and maltose.
Here are the equations for the disaccharides:
-Alpha glucose + Alpha glucose=Maltose
-Alpha glucose + Fructose=Sucrose
-Alpha glucose + Galactose=Lactose
Example question: Using a labeled diagram show the formation of maltose. [2]
As you can see here a dimer (chain with two monomer units) of maltose is formed with the Oxygen in the middle. In the first reaction oxygen and two hydrogens are given away as H2O or Water. Hence, leaving only oxygen. This is called a 1,4 glycosidic linkage as the bonds are formed between the first and fourth carbon.
3. Polysaccharides: They are very long chain of carbohydrate made up of many more monosaccharide molecules. Polysaccharides have functions such as energy storage (glycogen, starch) and structural maintenance (cellulose). They vary in solubilities based on the amounts of glycosidic linkages present. The main types of polysaccharides are starch, glycogen and cellulose.
Starch: it is the stored form of energy within plants and is mainly found in amyloplasts of plants. It is divided into two types amylose and amylopectin. The reason why plants do this to store energy is because it’s insoluble in water. The insolubility of water makes sure that the osmotic issues are not gonna happen in plants.
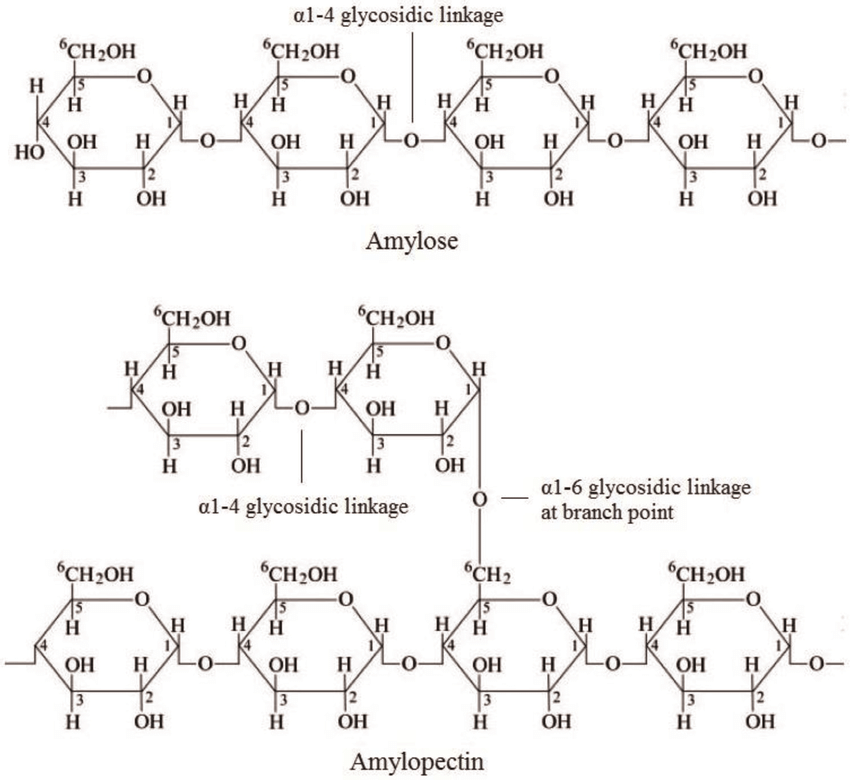
This outlines the bonds present in both.
Amylose: It has a linear structure and is unbranched. It is made up of alpha glucose molecules with 1,4 glycosidic linkages similar to that of maltose (don’t get into the misconception that those two are similar) but longer chains of alpha glucose than maltose. Its linear structure which lacks the branching allows for it to pack together (compact). It forms a helical structure allowing it to be packed and compressed.
Amylopectin: It has a branched structure. It has 1,4 glycosidic linkages between the alpha glucose molecules and has 1,6 glycosidic linkages in the branching points (it means there is a linkage between the first carbon atom of one glucose and sixth carbon atom of another glucose). The branching allow a rapid access of glucose for enzymes during metabolism making it more readily available as an energy source compared to amylose.
Cellulose: It is a charbohydrate that is found in cell walls of plants which is made from beta glucose monomers. Its composed of long chains of glucose molecules linked together.
The arrangement of glucose molecules in cellulose chains creates a strong, linear structure, making cellulose fibers tough and resistant to stretching. Hydrogen bonds form between adjacent cellulose molecules, providing additional strength and stability to the overall structure. Cellulose is insoluble in water, which prevents it from being easily broken down or degraded, enhancing its durability and longevity in plant cell walls.
Example Question!
Q1. Draw the formation of cellulose and explain how its feature is adapted for structural support. [3]
one important point of drawing a condensation reaction of cellulose is that the second Beta glucose molecule has to be flipped so that the OH groups has to be aligned. Adaptive features: 1.glucose arrangement makes it tough, 2.hydrogen bonds between adjacent cellulose giving strength and 3.insolubility.
Glycogen: This is the stored form of energy for animals. The glycogen is usually stored in the liver and muscle cells of t animals. Similar to amylopectin it also is for quick and accessible energy source.
As you see glycogen and amylopectin have a similar general structure except that it has more branches. This also means that more 1,6 and 1,4 glycosidic bonds are there making it more soluble than amylopectin.
Q2. What is the opposite reaction of condensation?
1.Electrolysis
2.osmosis
3.deposition
4.hydrolysis
ans: option 4 is the right answer as condensation is a reaction where the water molecules are lost and the molecules become a large polymer so its an anabolic reaction. However hydrolysis is a catabolic reaction which water is added and is broken down into sub units or smaller molecules.

Check out our other blogs on Biology here. Thanks for reading!



